The Chemistry of Water Chemistry for Life
Protein Structure
|
 |
Matter and Energy |
Think about it - What are you made of? Just as buildings are made from bricks, steel, glass, and wood, living things are made from chemical compounds.
![]() Key Questions Include:
Key Questions Include:
What three subatomic particles make up atoms?
How are all of the isotopes of an element similar?
In what ways do compounds differ from their component elements?
What are the main types of chemical bonds?
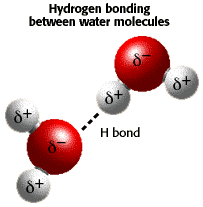
How does the structure of water contribute to its unique properties?
Why is it important for cells to buffer solutions against rapid changes in pH?
What elements does carbon bond with to make up life's molecules?
What are the functions of each of the four groups of macromolecules?
How do energy changes affect whether a chemical reaction will occur?
What role do enzymes play in living things and what affects their function?
The Ghost Fish
Several species of Antarctic fish have clear, almost transparent blood, giving them a ghostly appearance. Why? Because they lack hemoglobin, the oxygen-carrying protein that gives blood its red color. How do they manage to survive without this essential protein?
Click Here for the complete Chapter Mystery