Parasites and Drugs
Parasites and Drugs
Behe’s “Edge” argument rests on two basic points. The first is that a beneficial, selectable trait like chloroquine resistance can arise only after multiple, simultaneous mutations emerge at random. The target for those mutations is the gene for PfCRT, a membrane transport protein. In chloroquine resistant strains, mutant versions of this protein are able to pump the drug out of the cell’s digestive vacuole, enabling the parasite to survive. As he puts it, the data argue “that a first, required mutation to PfCRT is strongly deleterious, while the second may partially rescue the normal, required function of the protein, plus confer low chloroquine transport activity." Since that first “required” mutation is so deleterious, it couldn’t possibly spread through the population while waiting for the second to appear. Natural selection would weed out the deleterious mutation unless the second one popped up beside it in the same organism. That, according to Behe, accounts for the very low frequency of chloroquine resistance and validates his analysis.
Quite frankly, he must be secretly hoping that nobody actually looks at the details in the PNAS paper.
There is indeed one required mutation in the PfCRT protein, which is a change of an amino acid at position number 76 from lysine to threonine (see Figure 3). In the language of protein chemistry, that’s a K76T mutation (“K” stands for lysine, a positively charged amino acid, and “T” for threonine, which is uncharged).
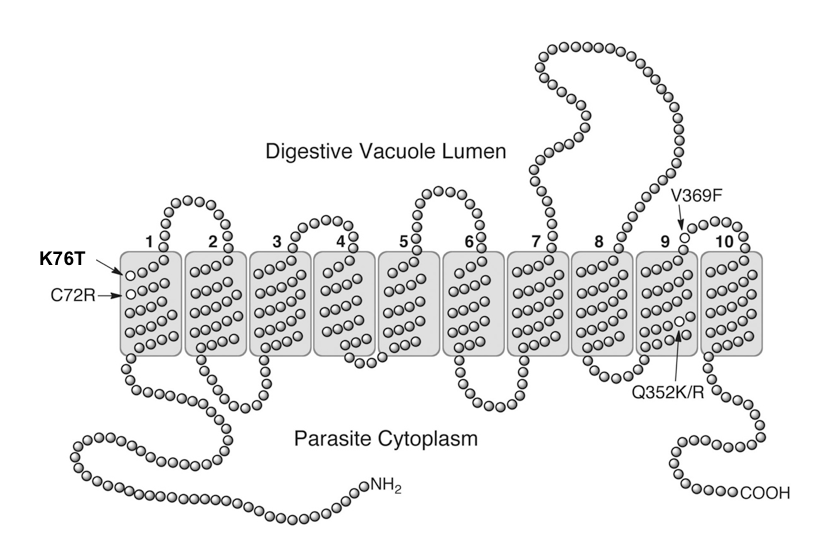
Figure 3: Diagrammatic view of the PfCRT protein, which spans the membrane of the parasite’s digestive vacuole. The position of the critical K76T mutation is noted, as are several other mutations known to occur in the protein. (Source: Griffin et al, 2012).
But Behe was dead wrong about it being “strongly deleterious.” In fact, it seems to have no effect on transport activity at all. A neutral mutation like this can easily propagate through a population, and field studies of the parasite confirm that is exactly what has happened. In fact, a 2003 study recommended against using the K76T mutation to test for chloroquine resistance since that same mutation was also found in 96% of patients who responded well to chloroquine. Clearly, K76T wouldn’t have become so widespread if it were indeed “strongly deleterious,” as Behe states it must be. This is a critical point, since Behe’s probability arguments depend on this incorrect claim.
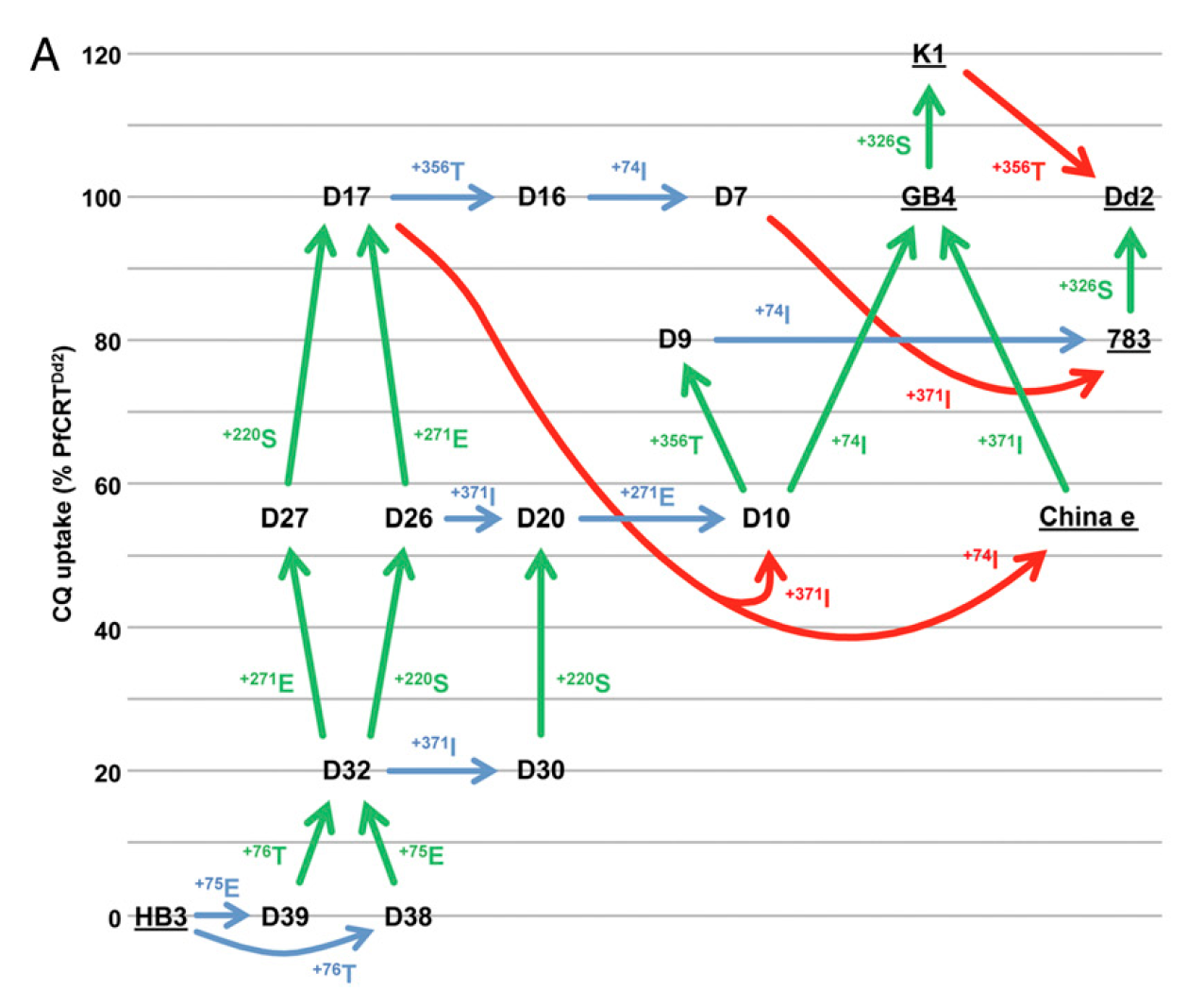
Directly contradicting Behe’s central thesis, the PNAS study also showed that once the K76T mutation appears, there are multiple mutational pathways to drug resistance. In most of these, each additional mutation is either neutral or beneficial to the parasite, allowing cumulative natural selection to gradually refine and improve the parasite’s ability to tolerate chloroquine. One of those routes involves a total of seven mutations, three neutral and four beneficial, to produce a high level of resistance to the drug. Figure 4, taken from the Summers et al PNAS paper, makes this point in graphic fashion, showing the multiple mutational routes to high levels of transport, which confer resistance to chloroquine.
Figure 4: Mutational pathways from the unmodified PfCRT protein (strain HB3) plotted against their ability to transport chloroquine. Neutral mutations are shown in blue, those that decrease transport in red, and the beneficial mutations that increase transport are shown in green.
(Click on Image to enlarge)
Pathways of this sort, involving sequential mutations, are exactly what Behe had tried to rule out, as I wrote in my own review of his book in 2007:
Behe obtains his probabilities by considering each mutation as an independent event, ruling out any role for cumulative selection, and requiring evolution to achieve an exact, predetermined result. Not only are each of these conditions unrealistic, but they do not apply even in the case of his chosen example. First, he overlooks the existence of chloroquine resistant strains of malaria lacking one of the mutations he claims to be essential (at position 220). This matters, because it shows that there are several mutational routes to effective drug resistance. Second, and more importantly, Behe waves away evidence suggesting that chloroquine resistance may be the result of sequential, not simultaneous, mutations.
We now know, courtesy of the PNAS paper, that such criticisms were right on target. There are indeed several mutational routes to drug resistance, and they are indeed the result of sequential, not simultaneous mutations. This matters because the assumption of simultaneous mutations is at the very heart of Behe’s math. That’s how he justifies multiplying one probability times another times another to conclude that complex traits are beyond the reach of the evolutionary process. To put it clearly, the problem with the logic of The Edge isn’t the specific figure of one chance in 1020, but the way in such probabilities are multiplied. In fact, it doesn’t really matter if chloroquine resistance emerges at a probability of one chance in 1020, one in 1015, or even one chance in 1010. The problem is the logic that Behe uses to calculate the chances of evolution producing two or more CCCs. As we will see, that’s the most critical part of his argument — and it’s wrong.
< Previous ..... Next >